Study with Quizlet and memorize flashcards containing terms like Which of these groups of particles has the greatest mass? a) A helium nucleus with two protons and two neutrons b) four electrons c) four individual protons, Which of these layers of the sun is coolest? a) Core b) Radiation zone c) photosphere, X-ray images of the sun generally show the
Brownness of Organic Aerosol over the United States: Evidence for Seasonal Biomass Burning and Photobleaching Effects | Environmental Science & Technology
31 Which of these particles has the greatest mass? (1) alpha (3) neutron (2) beta (4) positron. 1: Alpha then neutron then beta/positron: 32 In a nuclear fusion reaction, the mass of the products is (1) less than the mass of the reactants because some of the mass has been converted to energy (2) less than the mass of the reactants because some

Source Image: newatlas.com
Download Image
Which of these groups of particles has the greatest mass? (a) a helium nucleus with two protons and two neutrons (b) four electrons (c) four individual protons The correct answer is (c). You can add up the mass of 4 individual protons and compare them to the mass of a helium nucleus, and you nd that helium
Source Image: quora.com
Download Image
Understanding the Electrical Conductivity of Graphene | by Sasha Przybylski | Medium Sep 10, 2023Final answer: The group of particles with the greatest mass would be a helium nucleus with two protons and two neutrons. This is because protons and neutrons have more mass than neutrinos or photons, and a helium nucleus consists of 4 such particles. Explanation:

Source Image: slideshare.net
Download Image
Which Of These Groups Of Particles Has The Greatest Mass
Sep 10, 2023Final answer: The group of particles with the greatest mass would be a helium nucleus with two protons and two neutrons. This is because protons and neutrons have more mass than neutrinos or photons, and a helium nucleus consists of 4 such particles. Explanation: Video Transcript. List the following particles in order from the least mass to the greatest mass: tauon, photon, proton, electron, muon, neutron. We’ll be ranking our six particles from least mass to greatest mass. Of the six particles listed, we know that a photon is considered to be massless. Therefore, it would have the least mass out of
Kinetics of particles work energy method | PDF
Chapter 8 Due 11/16 1. Which of these groups of particles has the greatest mass? (a) four electrons (b) four individual protons (c) a helium nucleus with two protons and two neutrons 2. Compared with Earth’s diameter, the Sun’s diameter is about (a) the same; (b) 10 times larger; (c) 100 times larger; (d) 1 million times larger. 3. Atoms, Molecules, And Compounds. An element is formed of many similar… | by Bio Chemistry | Medium
Source Image: medium.com
Download Image
Starter: Which of these has the greatest mass and why? – ppt video online download Chapter 8 Due 11/16 1. Which of these groups of particles has the greatest mass? (a) four electrons (b) four individual protons (c) a helium nucleus with two protons and two neutrons 2. Compared with Earth’s diameter, the Sun’s diameter is about (a) the same; (b) 10 times larger; (c) 100 times larger; (d) 1 million times larger. 3.

Source Image: slideplayer.com
Download Image
Brownness of Organic Aerosol over the United States: Evidence for Seasonal Biomass Burning and Photobleaching Effects | Environmental Science & Technology Study with Quizlet and memorize flashcards containing terms like Which of these groups of particles has the greatest mass? a) A helium nucleus with two protons and two neutrons b) four electrons c) four individual protons, Which of these layers of the sun is coolest? a) Core b) Radiation zone c) photosphere, X-ray images of the sun generally show the

Source Image: pubs.acs.org
Download Image
Understanding the Electrical Conductivity of Graphene | by Sasha Przybylski | Medium Which of these groups of particles has the greatest mass? (a) a helium nucleus with two protons and two neutrons (b) four electrons (c) four individual protons The correct answer is (c). You can add up the mass of 4 individual protons and compare them to the mass of a helium nucleus, and you nd that helium
Source Image: medium.com
Download Image
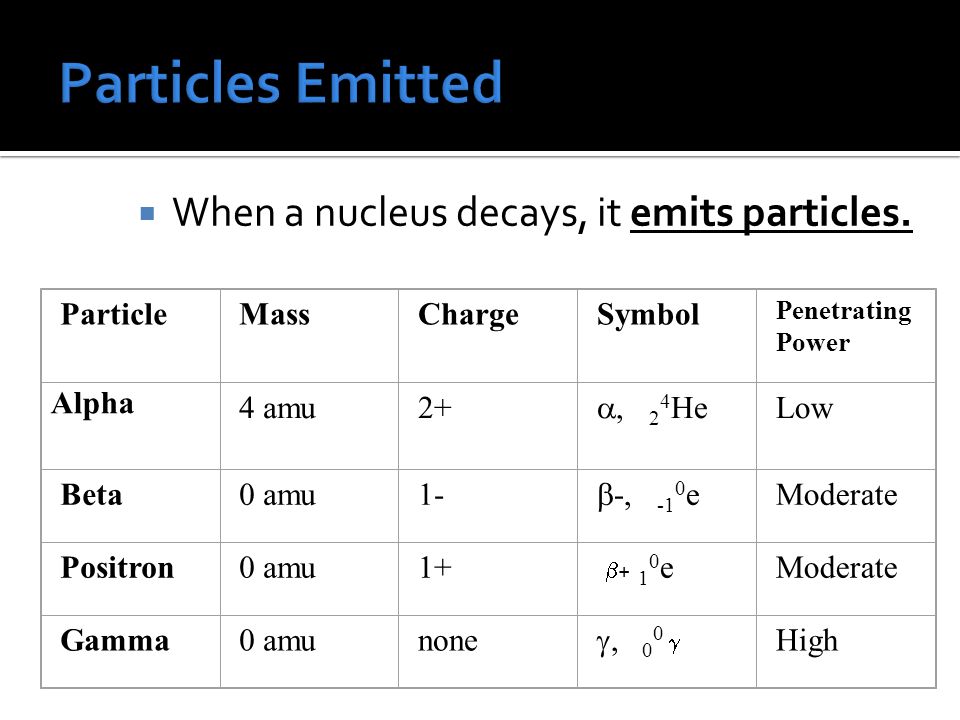
Isotope: same element (same #P) different number of neutrons (different mass #) Carbon-12 has 6 protons and 6 neutrons Carbon -14 has 6 protons and. – ppt download (b) Four electrons: – The mass of an electron is approximately 0.0005 amu. So, the mass of four electrons is 4 * 0.0005 amu = 0.002 amu. Step 2/2 (c) Four individual protons: – The mass of a proton is approximately 1 amu. So, the mass of four individual protons is 4 * 1 amu = 4 amu. Now, let’s compare the masses: – Group (a) has a mass of 4 amu.
Source Image: slideplayer.com
Download Image
Animal source foods in ethical, sustainable & healthy diets: Livestock and greenhouse gas emissions: 10 arguments for more nuance Sep 10, 2023Final answer: The group of particles with the greatest mass would be a helium nucleus with two protons and two neutrons. This is because protons and neutrons have more mass than neutrinos or photons, and a helium nucleus consists of 4 such particles. Explanation:

Source Image: aleph-2020.blogspot.com
Download Image
The Rise and Fall of Supersymmetry | by Ethan Siegel | Starts With A Bang! | Medium Video Transcript. List the following particles in order from the least mass to the greatest mass: tauon, photon, proton, electron, muon, neutron. We’ll be ranking our six particles from least mass to greatest mass. Of the six particles listed, we know that a photon is considered to be massless. Therefore, it would have the least mass out of

Source Image: medium.com
Download Image
Starter: Which of these has the greatest mass and why? – ppt video online download
The Rise and Fall of Supersymmetry | by Ethan Siegel | Starts With A Bang! | Medium 31 Which of these particles has the greatest mass? (1) alpha (3) neutron (2) beta (4) positron. 1: Alpha then neutron then beta/positron: 32 In a nuclear fusion reaction, the mass of the products is (1) less than the mass of the reactants because some of the mass has been converted to energy (2) less than the mass of the reactants because some
Understanding the Electrical Conductivity of Graphene | by Sasha Przybylski | Medium Animal source foods in ethical, sustainable & healthy diets: Livestock and greenhouse gas emissions: 10 arguments for more nuance (b) Four electrons: – The mass of an electron is approximately 0.0005 amu. So, the mass of four electrons is 4 * 0.0005 amu = 0.002 amu. Step 2/2 (c) Four individual protons: – The mass of a proton is approximately 1 amu. So, the mass of four individual protons is 4 * 1 amu = 4 amu. Now, let’s compare the masses: – Group (a) has a mass of 4 amu.